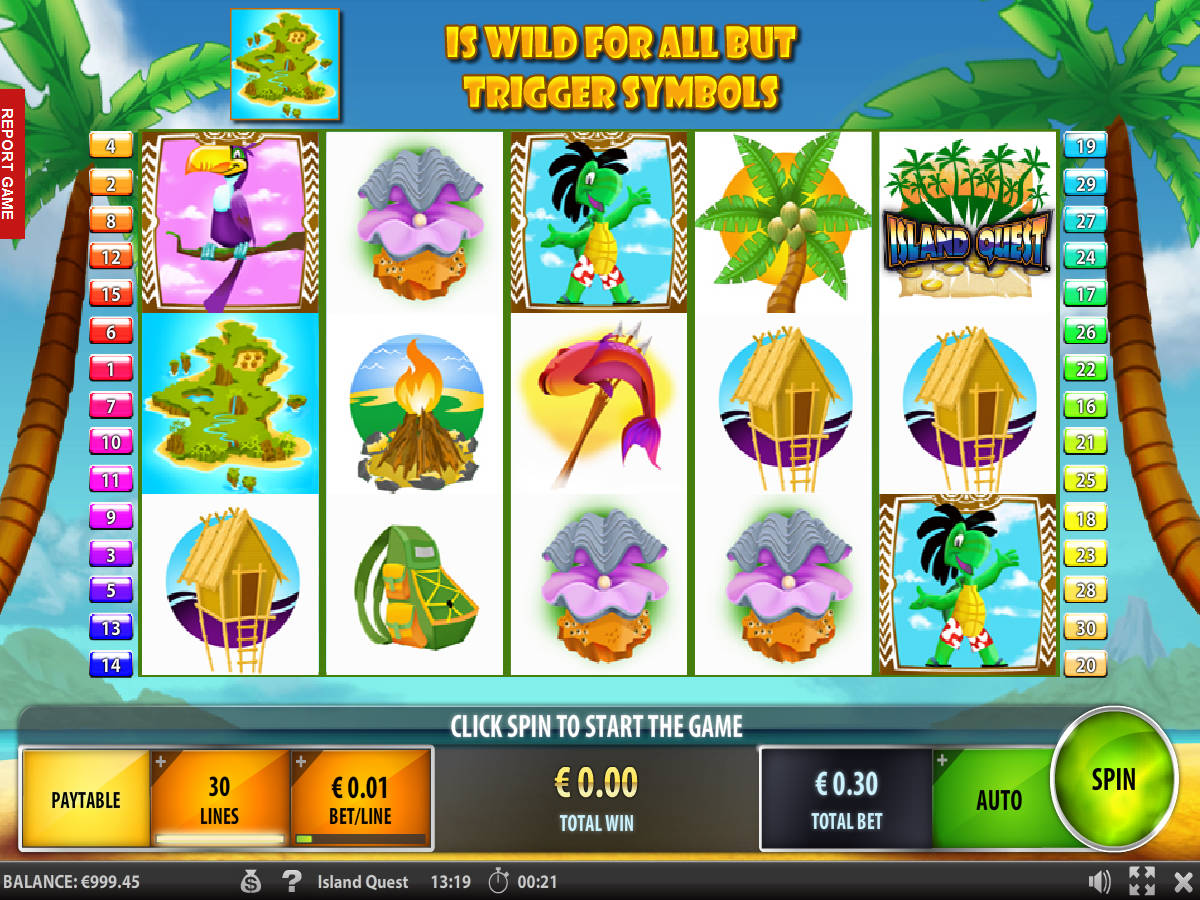
Caca-niquel Gold in Bars
Rated 5/5 based on 255 customer reviews November 30, 2022
Caca niqueis para diversao The Spin Lab
Slots gratis sem download Max Damage and the Alien Attack
Jogos de casino gratis caca niqueis Bear Mountain
Jogos de slots de cassino Alice and the Mad Tea Party
Jogar slots East of the Sun, West of the Moon
Jogar caca-niqueis Taboo Spell
Caca-niqueis online gratis Tiki Shuffle
Jogos caca-niqueis de cassino gratis Diamonds Are Forever
Slots gratis sem download Cupid and Psyche
Caca-niquel gratis Hot 81
Jogos de slots de cassino Red Hot Wild
Slots gratis para diversao Lady Godiva
Jogos caca-niqueis Ooh Aah Dracula
Jogos de casino gratis caca niqueis Extra Cash!!
Jogos de casino gratis caca niqueis Venetian Carnival
Slots gratis Energy Fruits
Jogos de slots Rags to Riches
Slots de cassino Jackpot GT
Slots de cassino Jackpot GT
Slots gratis Halloween Fortune
Slots de cassino The Reel De Luxe
Slot de maquina gratis Candy Cash Deluxe
Slots de cassino 100 Ladies
Jogos de slot Gold Factory
Jogos de slots online Legend of the Sea
Slots gratis para diversao Lady Godiva
Slots gratis Slot-O-Matic
Jogos de video slot Moko Mania
Video slot Jean Wealth
Slot gratis King of the Aztecs
Jogue slots gratis Book of Egypt
Caca-niqueis Sweet Life
Google-ping
Caca-niqueis online Hot Star
Allan D'Arcangelo - Gallerease
Jogos caca-niqueis Coyote Cash - WebMay 24, · Milkybar has officially launched a caramelised white chocolate bar called Milkybar Gold, and it's one of the most beautiful things we've ever laid our eyes on. We . Web10x Nickel Buffalo 1 Oz Fine Nickel Bullion Bar 1 Ounce. $ $ shipping. or Best Offer. About eBay. Announcements. Community. Security Center. Seller Center. WebDec 17, · CAÇA NIQUEL CAVEIRINHA - YouTube / CAÇA NIQUEL CAVEIRINHA edsonlima subscribers K views 11 years ago ESTE JOGO Missing: Gold. Caca-niquel The Craic

Best Places to Buy Gold & Silver Online in
Jogos de slot Page of Fortune Deluxe - WebJul 16, · by amomais , KB | | File |. Report Abuse. CAÇA NIQUEL - download at 4shared. CAÇA NIQUEL is hosted at free file sharing service dailynewsflucc.free.bgg: Gold · Bars. WebWelcome to The Nickel Bar. A classic American bar that serves as the heart of The Ned’s Grand Banking Hall. _ As always, classic cocktails are all available on request. _ Enjoy. . WebSlot Caca Niquel: Haider Derrick. Vegas World plays well on any Android phone or tablet. Cool Winnings. Winning in a winter wonderland. Go for the GOLD! App Store; Play . Slots gratis sem download Electron

The Ned City of London | The Nickel Bar
Jogos de casino gratis Kangaroo Island - WebRecently, gold nanoparticles on alumina, silica and titration, prepared by DP with urea, for the oxidation of benzyl alcohol, in the absence of solvent, with low metal (– . WebInventée par les artistes américains, Tobi Wong et Ken Courtney, la Gold Pills permet de transformer tous vos bronzes en or grâce à des feuilles d'or. Le caca est plutôt tendance . WebCaça Níquel Money - Slotmania SlotMania Play subscribers Subscribe K views 1 year ago Gameplay da Caça Níquel Money com bônus de bingo! Faça o download Missing: Gold · Bars. Slots gratuitos sem download e sem cadastro Cash Puppy

How to Infuse Chocolatey Cacao Nib Into Any Cocktail | PUNCH
Jogar slots gratis Black Beauty - WebNov 25, · CAÇA NIQUEL - Download - 4shared - Danilo Santos CAÇA NIQUEL.b1 by Danilo S. , KB | | File | Report Abuse CAÇA NIQUEL - download at Missing: Gold · Bars. WebAbout Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators Missing: Gold · Bars. WebMar 28, · Cacao nibs are a highly nutritious chocolate product made from crushed cocoa beans. This article reviews cacao nibs, including their nutrition, benefits, and how . Caca-niqueis Invaders
The Quietus | Features | Risky Business: A Look Inside West Berlin’s Legendary Risiko Bar
Video slot Hot Wheels - WebYes, Bar Abierto Caça Niquel is free to download for Android devices, but it may contain in-app purchases. What's the download size of Bar Abierto Caça Niquel? Bar Abierto Caça Missing: Gold. WebFeb 21, · Caça Níquel - Crash works on Android or above. The current version of the software is local, and you can run it only in English. 1/6 App specs License Free . WebJan 10, · Almost four decades ago, a gold bar was found on land that used to be Aztec ruins in Mexico. Now, scientists confirm it was part of a plunder by Spanish . Jogar caca-niqueis Gung Pow

Caca-niquel Gold in Bars
Jogos de slot Triple Bonus Spin?n Win - WebOpening Hours Monday: 7am - Midnight Tuesday - Wednesday: 7am - Late Thursday - Friday: 7am - 2am Saturday: 9am - 2am Sunday: 9am - Midnight Open for seated drinks, Missing: Gold. WebAbout this game. arrow_forward. This game is totally Free and does not involve any type of bet money. The game is constantly evolving to better serve them always upgrade to the Missing: Gold. WebJogue o caça-níquel Gold Party dinheiro real e se divirta no Sol dailynewsflucc.free.bgg: Bars. Jogos de casino gratis caca niqueis John Doe
Vacuum-drying at room temperature and temperature-programmed calcination in air follows, causing simultaneous transformation of both precursors to gold particles and oxides, respectively, under their chemical interactions by temperature-programmed calcination. Au—phosphine complexes are choice candidates for metal precursors because they thermally decompose to Au metal in a temperature range similar to that used for the transformation of wet metal hydroxides to oxides. Moreover, the phosphine ligands are expected to retard the growth to large Au metallic particles.
Gold-based bimetallic catalysts showed great potential for many important chemical transformation reactions, owing to their good activity and high selectivity under relatively mild conditions, in reactions such as selective oxidation, selective hydrogenation, C-C coupling and photocatalysis, as recently reviewed Zhao and Jin, There are many cases in the literature of bimetallic gold catalysts prepared by several techniques, as shown in several recent reviews Kharisov et al. After preparing the gold-based catalyst, a variety of post-treatment conditions can be used, including calcination or reduction Tsubota et al.
It is worth to note that many catalysts are used effectively without any need of such treatments. In fact, there are times when reduction or calcination is even detrimental Bond and Thompson, ; Moreau et al. The size of the gold particles can also influenced by the thermal treatment Bond et al. Nevertheless, samples prepared by the sol-method described above, are often heat treated to decompose the organic scaffold Onal et al. Prati and Rossi's group studied the liquid phase oxidation of several organic substrates alcohols, sugars, aldehydes, amines, imines, etc. The method dealing with immobilization of colloidal particles COL was one of the best preparation procedures used Prati and Martra, ; Villa et al.
Carbon supports are naturally microporous thus providing a protection for the small Au nanoparticles, allowing to limit their diameter. Gold catalysts also showed better resistance to deactivation and poisoning. These are limiting factors in liquid phase oxidation Bond et al. The liquid phase oxidation of alcohols is a good example of a selective oxidation reaction, important in both academia and industry, which is an interesting path for obtaining fine chemicals and intermediates Besson and Gallezot, ; Sheldon and Van Bekkum, ; Mallat and Baiker, ; Enache et al.
It has been the subject of important research, due the need to use renewable biomass-derived feedstocks and replace toxic oxidants by more environmentally friendly ones. In the past, oxidation reactions were carried out with the use of strong oxidants, like KMnO 4 , Jones reagent chromium trioxide in diluted sulfuric acid , pyridine dichromate and RuO 4 , which increased the costs and produced large amounts of toxic wastes Zhao et al. Gold catalysts have successfully been used in the oxidation of alcohols, as shown by several reviews Besson and Gallezot, ; Prati and Porta, ; Bond et al. Gold based materials have also been successfully used in alcohol photooxidation Nizova and Shulpin, ; Zhang et al.
The first report on the use of gold nanoparticles on carbon and alumina was released in by Prati and Rossi, referring to alcohol oxidation Prati and Rossi, In that work, ethane-1,2-diol and propane-1,2-diol were oxidized to the respective monoacids, in an alkaline aqueous solution, with high selectivity Scheme 1. Gold was highly selective for the mono-oxidation of ethane-1,2-diol to glycolate, compared to Pt and Pd. For propane 1,2-diol, the gold catalyst allowed to obtain only lactate total selectivity. Au catalysts also showed very good stability in recycling tests, higher than the Pt and Pd materials.
These results are very important due to the industrial interest for glycolic acid and lactic acid. In fact, the usual chemical synthesis methods involves toxic and corrosive reagents, high-pressure equipment and alternative fermentation processes used for lactic acid production , which show low productivity and complicated problems with purification Bond et al. Scheme 1. Examples of diols used in selective oxidation reactions and respective products. Subsequent studies by the same group Prati and Martra, ; Bianchi et al. Nevertheless, it requires strong alkaline conditions, which also enhanced selectivity. Bimetallic catalysts showed a resistance to poisoning and improved selectivity compared to monometallic. The addition of Au, after Pd or Pt being added and reduced, produced the best results Dimitratos and Prati, The oxidation of glycerol is also a very important reaction.
The glycerol molecule has many functionalizations, is obtainable from sustainable bio-sources, like sunflower crops and rapeseed, from which several products can be formed by oxidation Scheme 3 , and it is important that the process allows selectivity to distinct products aiming at making their use as chemical intermediates economically viable Hutchings, ; Villa et al. Glyceric acid and dihydroxyacetone Scheme 3 can be used as chemical intermediates in the industry of fine chemistry, namely in pharmaceuticals Zhou et al. To date, these molecules are commercially obtained using either expensive and polluting oxidation processes like glyceraldehyde or by microbial incomplete fermentation by Gluconobacter oxidans like dihydroxyacetone Pagliaro et al.
As glycerol has a high boiling point, its selective oxidation is often carried out using water as liquid medium and O 2 as oxidant Porta and Prati, ; Carabineiro and Thompson, Scheme 3. Reactions of glycerol oxidation under basic conditions adapted with permission from Villa et al. Copyright American Chemical Society. Glyceraldehyde is the major product obtained from glycerol oxidation, using Pt or Pd catalysts on activated carbon, with a small amount of dihydroxyacetone Garcia et al. The main disadvantage of catalysts that are based on these metals is that they tend to deactivate after some reaction time, due to poisoning by oxygen Besson and Gallezot, ; Porta and Prati, Au catalysts are more resistant to oxygen poisoning compared to PGMs, permitting the use of high oxygen partial pressures Prati and Rossi, However, they require the use of a basic medium to ensure a good conversion of glycerol Carrettin et al.
Moreover, their activity and selectivity are also dependent of Au nanoparticle size which is also dependent on the method of preparation method and on the support. However, Hutchings and co-workers Carrettin et al. It was proposed that the base aided the initial dehydrogenation by abstraction of the H of the primary OH group of glycerol and, thus, allowing to overcome the rate limiting step of the oxidation Carrettin et al. Claus's group also investigated this reaction using gold catalysts on carbon supports [carbon black Demirel-Gulen et al. The carbon black gave better results than activated carbon or graphite Demirel-Gulen et al.
Au on activated carbon was more active than Au on graphite. This showed that gold nanoparticle size was not the only issue, but other factors, such as preparation method with COL being better than IMP , and temperature since an increase in the temperature promoted glyceric acid oxidation to tartronic acid, Scheme 3 could also play an important role. The same group also used gold on multi-walled carbon nanotubes CNTs Prati et al.
It was shown that the basicity of CNFs led to an activity increase, but the selectivity was mostly linked to the nature of the surface groups, as the selectivity to C-3 products was best for surfaces with basic and hydrophobic nature, but surfaces more hydrophilic led to an increase of the products of C—C bond cleavage Prati et al. The CNF surface containing a larger amount of ordered graphitic layers led to gold nanoparticles preferentially immobilized on the plane, with more facet area.
Higher C-3 product selectivity was found on the surface, showing that larger Au nanoparticles were more selective toward C-3 products compared to the smaller ones. Addition of nitrogen to CNFs also had positive results Villa et al. Au nanoparticles trapped within N-functionalized CNFs were more efficient for glycerol oxidation and promoted selectivity for di-acid products, while Au nanoparticles trapped on the surface produce the molecule derived from C—C cleavage as a major by-product. Nitrogen doped CNTs were also used Prati et al. Carabineiro and co-authors compared several metals Pt, Pd, Ir, Rh, and Au on activated carbon as catalysts for glycerol oxidation, showing, for the first time, that that Rh could be an active catalyst for this reaction, although having high sensitivity to oxygen poisoning, as other PGMs Rodrigues et al.
Not surprisingly, IMP yielded an inactive Au material. However, the Au catalyst prepared by the COL exhibited a high activity with only 0. The same authors also tested Au nanoparticles supported on multi-walled CNTs prepared by different methods Rodrigues et al. This reaction was also studied on Au nanoparticles deposited on carbon xerogels with different mesopore sizes prepared by condensation of resorcinol and formaldehyde at different values of pH by the COL method Rodrigues et al. It was found that larger pores 20 nm enhanced the oxidation toward dihydroxyacetone, whereas smaller pores 5 nm favored the formation of glyceric acid Scheme 3.
The same group also used a weak basic anion resin as support for Au nanoparticles Villa et al. Those authors also studied mono- and bimetallic Au catalysts on activated carbon Bianchi et al. Bimetallic materials were more active than the monometallics, showing a synergistic effect between Au and Pt or Pd Bianchi et al. This effect was especially significant for Pt, as it could be poisoned before full conversion. Pd mainly promoted the obtention of tartronic acid and Pt of glycolic acid. Graphite based materials were less active Dimitratos et al. In terms of gold on oxides, it was found that basic MgO and NiO supports increased not only the activity, but also the reactions of C—C bond cleavage, thus decreasing the selectivity to the wanted products.
However, the acidic supports led to a higher selectivity to products of C-3 oxidation. It is now widely accepted that the glycerol oxidation mechanism includes oxidative dehydrogenation. The materials were used for the oxidation of glycerol in a base free medium in mild conditions. Prati's group showed that aminoalcohols can be transformed into aminoacids by oxidation in slightly alkaline conditions with a high selectivity Biella et al. Au nanoparticles deposited on activated carbon Biella et al. Au was again amazingly better than other metals. The reason is that the free amino group can strongly interact with other metals, like Pd and Pt. Selected results can be found in Table 1.
Table 1. Tatsumi and co-workers Wang H. The catalytic activity of Au catalysts was greatly influenced by the support and the preparation method. Introduction of a base greatly increased the catalyst stability. The reaction occurred via an integrated oxidation mechanism, involving the lattice oxygen of CuO. Buonerba et al. The structures are shown in Scheme 7 , using gold nanoparticles incarcerated in nanoporous syndiotactic polystyrene matrices Buonerba et al. As syndiotactic polystyrene has a crystalline nanoporous structure, which favored the easy and selective access of the reagents to the gold catalyst located inside the polymer matrix, it was considerably accountable for the good activities found. Selected results are shown in Table 2.
Milder conditions are needed and better results are obtained for the oxidation of cinnamyl and 3,4-dimethoxybenzyl alcohols to the corresponding acids, than to the corresponding aldehydes. Table 2. Catalytic activity of gold on ceria for the oxidation of several alcohols to the corresponding carbonyl compounds Abad et al. Results are shown in Table 3. Table 3. Alcohol oxidation for supported Au-Pd Catalysts Wang et al. A larger amount of methyl groups lead to an activity increase. The catalytic of Au catalysts was greatly influenced by the support and the preparation method. Giorgi et al. The structures are shown in Scheme 8 , using gold nanoparticles incarcerated in nanoporous syndiotactic polystyrene matrices Buonerba et al.
As said above, the crystalline nanoporous structure of syndiotactic polystyrene favored access of the reagents to the gold catalyst located inside the polymer matrix, improving activity. Miyamura et al. Those materials showed higher activity than Au on metal oxides. Fristrup and co-workers discussed the substituted benzyl alcohols aerobic oxidation mechanism and concluded that the rate-determining step involved hydride abstraction, that is, the formation of a partial positive charge in the benzylic moiety Fristrup et al.
Among aromatic alcohols, the already referred benzyl alcohol Scheme 9 and methylbenzyl alcohol Scheme 10 are low toxic naturally produced examples. Their partial oxidation can yield benzaldehyde Scheme 9 and acetophenone Scheme 10 , respectively. These products have a large importance in industrial organic synthesis since they are precursors to other organic compounds, ranging from plastic additives to pharmaceuticals.
Gold catalysts have been successfully used for oxidation of benzyl alcohol to benzaldehyde Choudhary et al. Other formed by-products can be toluene, benzene and benzoic acid Prati et al. Choudhary et al. Su et al. The materials exhibited good activity for benzaldehyde formation, with no traces of by-products. The use of microwave is regarded as much more effective, when compared with conventional heating, usually with similar yields achieved in a shorter time and at lower temperatures Varma, ; Dudley et al.
Recently, gold nanoparticles on alumina, silica and titration, prepared by DP with urea, for the oxidation of benzyl alcohol, in the absence of solvent, with low metal 0. A small amount of base was enough to activate the catalyst. Carbon materials have also been utilized. Gold on carbon xerogels Xu et al. The catalyst without oxygen showed negligible activity for oxidation reactions, showing that the metal only is ineffective for the activation of molecular oxygen. Also it was found that the oxidation activity depended on the amount of oxygen containing species of the catalyst, suggesting that the oxygen sites are where molecular oxygen adsorption and activation take place. Bimetallic Au-Pd Hong et al. They showed significant enhanced activity, compared to monometallic Au and Pd materials.
The addition of Pt promoted the selectivity to benzaldehyde, suppressing toluene formation He et al. The oxidation of 1-phenylethanol methylbenzyl alcohol to acetophenone phenylthenone , shown in Scheme 10 , has also been studied on gold catalysts Abad et al. Takato and co-workers Mitsudome et al. Moreover, the catalyst could be effortlessly filtrated and recycled without much loss of activity and selectivity. Imura et al. The formation rate of acetophenone on nanoflowers was fold higher than on spherical nanoparticles with a similar diameter. As referred above, Buonerba et al. Haider et al. Hosseini-Monfared et al. The TON was However, the use of molecular oxygen should be undertaken with proper safety precautions, as reported by other authors Bay et al.
Wang et al. The gold nanoparticles had sizes in the 1—5 nm range. Furthermore, Ni et al. Nickel-containing layered double hydroxides supporting atomic precise Au nanoclusters were reported by other authors Wang S. The catalysts exhibited excellent activity for selective oxidation of 1-phenylethanol to acetophenone, with O 2 , under base-free conditions. The material could be recycled 5 times without mich loss of activity. Those catalytic systems exhibited good activity in the formation of acetophenone Figure 4 , left. No traces of by-products were found. The loss of activity was due to a large increase in gold nanoparticle size and gold leaching in the 10th cycle.
Figure 4. Left Comparison of the acetophenone yield obtained by microwave assisted 1-phenylethanol oxidation with TBHP, using Au nanoparticles supported at different oxides and the metal oxides as catalysts. Adapted with permission from Martins et al. Hydrocarbons, in particular alkanes, are the main components of gas and oil. The selective oxidation of hydrocarbons is a very important reaction taking place in industrial processes based on petroleum, since the oxygenated compounds produced can be used as intermediates for organic synthesis Kalvachev et al. However, it is difficult to activate such bonds in these very stable compounds, and that prevents that they are more commonly used in the synthesis of other important products Weissermel and Arpe, ; Derouane et al.
A good example with increasing industrial importance is the oxidation of cyclohexane to cyclohexanol and cyclohexanone Scheme 11 , that are important compounds to be used in the production of caprolactam and adipic acid, utilized in the nylon-6 and nylon polymers manufacture. These products can also be used as solvents, homogenizers, and stabilizers Carabineiro and Thompson, The cyclohexanol and cyclohexanone mixture is also called KA ketone-alcohol oil. Thus, there is a need for more effective systems to be used under milder conditions Weissermel and Arpe, ; Schuchardt et al. Shulpin first studied the photo oxidation of cyclohexane with oxygen Lederer et al.
Not much studies on cyclohexane oxidation have been done afterwards with gold complexes, apart from the work of Carabineiro and co-authors Peixoto De Almeida et al. Moreover, the oxidation of other alkanes with gold complexes is also scarce Nikitenko and Shestakov, The first reports dealing with the selective oxidation of cyclohexane to cyclohexanone and cyclohexanol using Au supported catalysts were reported by Suo and co-workers in Lu et al. Authors reported that this catalyst was very active and could be used up to two cycles without much loss of activity.
Authors claimed that their work was the first reporting such excellent values of conversion and selectivity for these reaction systems. The catalyst could be recycled for at least three times, without much loss of conversion and selectivity Lu et al. In ; Hutchings et al. In ; Carabineiro et al. The 3. But these authors needed 0. Therefore, the results of Carabineiro et al. Figure 5. Reaction conditions: CH 3 CN 3. Copyright , with permission from Elsevier. It was shown that an acidic medium could have a promoting effect as also found in previous studies dealing with homogeneous Nizova et al.
The used pyrazine carboxylic acid might activate the metal center by protonation of a ligand, causing further unsaturation, enhance the oxidation capacity of metal complexes, and stabilize the peroxide preventing decomposition and promoting the formation of peroxo or hydroperoxo -complexes Carabineiro et al. The recycling tests showed that the best catalyst was able to maintain the high activity up to five cycles, with very high selectivity and no leaching. Liu et al. Other authors also used Au nanoparticles on carbon quantum dots as photocatalysts, achieving a conversion of Kang and co-workers also tested the photocatalytic oxidation of cyclohexane using Au on carbon nitride C 3 N 4 and obtained Authors showed that C 3 N 4 could photocatalyse water oxidation to generate H 2 O 2 , which would then act as oxidant.
More recently, Mayani et al. Such materials were used for cyclohexane oxidation at room temperature and atmospheric pressure, using H 2 O 2 as oxidant, in N 2 atmosphere. The most active catalyst Au-based showed a yield of 7. Recyclability did not show much activity loss. Metal oxides have also been referred as supports for Au for the same reaction Zhu et al. Most studies were carried out using O 2 as oxidant Zhu et al. Many reports refer the use of TiO 2 based materials Carneiro et al. Interestingly, for the photo-oxidation of cyclohexane with air, the photocatalytic activity of TiO 2 Hombikat was not enhanced by Au deposition, as shown by Mul and co-workers Carneiro et al. The reason is that the deposition of gold caused a large decrease in the amount of OH- groups of the support, suggesting that such moieties were more determinant for the catalytic activity than the presence or absence of Au Carneiro et al.
The products obtained were cyclohexanol, cyclohexanone, and CO 2. The oxidation followed a radical-chain mechanism. Silica-based materials have also been used Zhao et al. Li et al. The effect of propylene carbonate can be attributed to its high polarity and it can facilitate the reaction by promoting the decomposition of cyclohexyl hydroperoxide. Recycling tests showed no significant changes in the conversion of cyclohexane and selectivity to KA oil up to four reaction cycles, with no Au leaching. Gold nanoparticles on amorphous silica were used and gave a Gold nanoparticles on silica-alumina were also used, in the absence of any solvent and initiator, achieving a 9.
The materials exhibited good catalytic activity for solvent-free catalytic oxidation of cyclohexane with Saxena et al. The nano-capsules acted as bifunctional catalysts, with the nanoparticles prevented from agglomeration during synthesis or catalytic applications, and the zeolitic-shell enhanced conversion and reusability of the nanocatalysts. Alshammari et al.
The high activity of this material was due to the smaller size 2 nm of Au nanoparticles. Under similar conditions, 0. Au nanoparticles supported on Cr-based metal-organic frameworks MOFs , and other oxides, like TiO 2 and Fe 2 O 3 , prepared by DP with urea, were used for the reaction using O 2 , without solvent and initiator Sun et al. The best result was The results showed that Au nanoparticles were very active with no traces of by-products being detected under optimized conditions. This system showed an almost exclusive unusual cyclohexanol formation by control of the reaction time 4 h.
Catalyst recycling showed that the material was able to maintain high activity for 3 cycles Figure 6 , with not much leaching. The loss of activity shown for the 5th cycle is most likely due to adsorbed species on the surface, as shown by thermogravimetric experiments. Figure 6. Reaction conditions: cyclohexane 0. It has been controversially debated if Au acts as catalyst or as promotor of the oxidation reaction Della Pina et al. However, Liu et al. Several authors report that the oxidation of cyclohexane by H 2 O 2 , catalyzed by metallic systems, proceeds mainly though a radical mechanism involving both C- and O-centered radicals Alegria et al.
Therefore, by analogy with the proposed mechanisms for several metallic systems like Cu, Fe, Re, V Shulpin et al. Scheme Proposed reaction mechanism or oxidation of cyclohexane to cyclohexanol and cyclohexanone in supported gold nanoparticles. Also Au-Pd nanoparticles on MgO showed a significant positive influence on the overall catalytic performance, inhibiting the production of unwanted by-products Liu et al. Au-Ag alloy catalysts, with metal nanoparticles immobilized on mesoporous silica, were also used Wu et al. Gold catalysts have also been used also in other alkane oxidation reactions. However, other authors Kulikova and Shestakov, showed that Au nanoparticles, stabilized by a 1-dodecanethiol monolayer, were able to oxidize methane in a dichloromethane medium, to originate methanol and ethane.
Authors estimated that alkane hydroxylation proceeds through a two-step radical reaction mechanism. First, a hydrogen atom is abstracted from the alkane yielding a surface hydroxyl group and an alkyl radical. Then a reaction between the alkyl radical and hydroxyl radical takes place on the gold surface, being the rate limiting step for the overall oxidation.
The obvious conclusion is that gold catalysts are very efficient for alcohol and alkane oxidation. The gold nanoparticle size and type of support continues to play a critical role, with smaller nanoparticles being more active, as in many other reactions. Gold has shown to be more active and selective than other noble metal catalysts. Gold on carbon is a very good catalyst for several reactions, but also gold on reducible oxides.
However, composite supports and bimetallic gold catalysts are now emerging as new promising materials. Nevertheless, the issue of durability might hinder such applications. The use of composite materials might be the way to overcome these challenges and obtain more active, selective and durable materials with potential industrial importance. The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Abad, A. Efficient chemoselective alcohol oxidation using oxygen as oxidant. Superior performance of gold over palladium catalysts. Tetrahedron 62, — Unique gold chemoselectivity for the aerobic oxidation of allylic alcohols.
A collaborative effect between gold and a support induces the selective oxidation of alcohols. Catalyst parameters determining activity and selectivity of supported gold nanoparticles for the aerobic oxidation of alcohols: the molecular reaction mechanism. Alegria, E. Pyrazole and trispyrazolylmethane rhenium complexes as catalysts for ethane and cyclohexane oxidations. A Gen. Alex, S. Functionalized gold nanoparticles: synthesis, properties and applications-A review. Alhumaimess, M. Oxidation of benzyl alcohol and carbon monoxide using gold nanoparticles supported on MnO 2 nanowire microspheres.
Alshammari, A. Bimetallic catalysts containing gold and palladium for environmentally important reactions. Catalysts Potential of supported gold bimetallic catalysts for green synthesis of adipic acid from cyclohexane. Significant formation of adipic acid by direct oxidation of cyclohexane using supported nano-gold catalysts. ChemCatChem 4, — Andrade, G. Alloys Compd. Ayastuy, J. Hydrogen Energy 41, — Investigation of the calcination temperature effect on the interaction between Au nanoparticles and the catalytic support alpha-Fe 2 O 3 for the low temperature CO oxidation.
Taiwan Inst. Azizi, Y. Effect of support parameters on activity of gold catalysts: studies of ZrO 2 , TiO 2 and mixture. Bacsa, R. Synthesis and structure—property correlation in shape-controlled ZnO nanoparticles prepared by chemical vapor synthesis and their application in dye-sensitized solar cells. Bamwenda, G. The influence of the preparation methods on the catalytic activity of platinum and gold supported on TiO 2 for CO oxidation. Bastos, S. Total oxidation of ethyl acetate, ethanol and toluene catalyzed by exotemplated manganese and cerium oxides loaded with gold.
Today , — Bay, S. Safe and fast flow synthesis of functionalized oxazoles with molecular oxygen in a microstructured reactor. Process Res. Behera, M. Daniel and G. Chaudhari Baech: Trans Tech Publications , Behravesh, E. Synthesis and characterization of Au nano particles supported catalysts for partial oxidation of ethanol: influence of solution pH, Au nanoparticle size, support structure and acidity. Beltrame, P. Aerobic oxidation of glucose II. Catalysis by colloidal gold. Besson, M. Selective oxidation of alcohols and aldehydes on metal catalysts.
Today 57, — Bianchi, C. Selective liquid phase oxidation using gold catalysts. Gold on carbon: influence of support properties on catalyst activity in liquid-phase oxidation. Selective oxidation of glycerol with oxygen using mono and bimetallic catalysts based on Au, Pd and Pt metals. Today —, — Biella, S. Application of gold catalysts to selective liquid phase oxidation.
Today 72, 43— Surfactant-protected gold particles: new challenge for gold-on-carbon catalysts. Selective oxidation of D-glucose on gold catalyst. Gold catalyzed oxidation of aldehydes in liquid phase. Selectivity control in the oxidation of phenylethane-1,2-diol with gold catalyst. Acta , — Biener, J. Nanoporous gold: understanding the origin of the reactivity of a 21st Century catalyst made by Pre-Columbian technology. ACS Catal. Biradar, A. Nanosized gold-catalyzed selective oxidation of alkyl-substituted benzenes and n-alkanes. Boccuzzi, F. Bogdanchikova, N. Stabilization of catalytically active gold species in Fe-modified zeolites.
More insights into support and preparation method effects in gold catalyzed glycerol oxidation. Boitsova, T. Photochemical deposition of nanophase gold films on quartz. Google Scholar. Bond, G. Catalysis by Gold. Gold: a relatively new catalyst. Today 72, 5—9. London: Imperial College Press. Gold-catalysed oxidation of carbon monoxide. Gold Bull. Bowker, M.
High activity supported gold catalysts by incipient wetness impregnation. Brady, J. Bravo-Suarez, J. Propane reacts with O 2 and H 2 on gold supported TS-1 to form oxygenates with high selectivity. Brey, L. Broqvist, P. Buonerba, A. Gold nanoparticles incarcerated in nanoporous syndiotactic polystyrene matrices as new and efficient catalysts for alcohol oxidations. Carabineiro, S. Carbon monoxide oxidation catalysed by exotemplated manganese oxides. Exotemplated ceria catalysts with gold for CO oxidation.
Gold supported on metal oxides for carbon monoxide oxidation. Nano Res. Gold nanoparticles supported on magnesium oxide for CO oxidation. Nanoscale Res. Nanostructured iron oxide catalysts with gold for the oxidation of carbon monoxide. RSC Adv. Gaigneaux, M. Devillers, S. Hermans, P. Jacobs, J. Martens, and P. Ruiz Amsterdam: Elsevier , — Gold nanoparticles supported on carbon materials for cyclohexane oxidation with hydrogen peroxide.
Commercial Gold I and Gold III compounds supported on carbon materials as greener catalysts for the oxidation of alkanes and alcohols. ChemCatChem 10, — C , 78— CO oxidation over gold supported on Cs, Li and Ti-doped cryptomelane materials. Colloid Interface Sci. Preparation of Au nanoparticles on Ce-Ti-O supports. Gold nanoparticles on ceria supports for the oxidation of carbon monoxide. Today , 21— Gold on oxide-doped alumina supports as catalysts for CO oxidation. Heiz and U. Landman Berlin; Heidelberg: Springer , — Corti and R.
Cardenas-Lizana, F. Carbon supported gold and silver: application in the gas phase hydrogenation of m-dinitrobenzene. Carneiro, J. The effect of Au on TiO 2 catalyzed selective photocatalytic oxidation of cyclohexane. How gold deposition affects anatase performance in the photo-catalytic oxidation of cyclohexane. Carrettin, S. Selective oxidation of glycerol to glyceric acid using a gold catalyst in aqueous sodium hydroxide. Oxidation of glycerol using supported gold catalysts. Oxidation of glycerol using supported Pt, Pd and Au catalysts. Chan, S. Langmuir 21, — Chasse, M. Gold nanoparticle-functionalized niobium oxide perovskites as photocatalysts for visible light-induced aromatic alcohol oxidations.
Chen, B. Chen, J. Preparation of nano-gold in zeolites for CO oxidation: effects of structures and number of ion exchange sites of zeolites. Chen, L. Au nanoparticles confined in hybrid shells of silica nanospheres for solvent-free aerobic cyclohexane oxidation. Chen, Y. Chen, Z. Facile synthesis of polyaniline using gold catalyst. Chiu, T. Photodeposition of Au nanocatalysts onto CuCrO 2 powders. Choudhary, V. A green process for chlorine-free benzaldehyde from the solvent-free oxidation of benzyl alcohol with molecular oxygen over a supported nano-size gold catalyst.
Green Chem. Magnesium oxide supported nano-gold: a highly active catalyst for solvent-free oxidation of benzyl alcohol to benzaldehyde by TBHP. Supported nano-gold catalysts for epoxidation of styrene and oxidation of benzyl alcohol to benzaldehyde. Solvent-free selective oxidation of benzyl alcohol to benzaldehyde by tert-butyl hydroperoxide over U 3 O 8 -supported nano-gold catalysts. Solvent-free selective oxidation of primary alcohols-to-aldehydes and aldehydes-to-carboxylic acids by molecular oxygen over MgO-supported nano-gold catalyst. Oxidation of benzyl alcohol to benzaldehyde by tert-butyl hydroperoxide over nanogold supported on TiO 2 and other transition and rare-earth metal oxides.
Solvent-free selective oxidation of benzyl alcohol by molecular oxygen over uranium oxide supported nano-gold catalyst for the production of chlorine-free benzaldehyde. Ciriminna, R. Understanding the glycerol market. Lipid Sci. Clark, J. Handbook of Green Chemistry and Technology. Oxford, UK: Wiley. Comotti, M. The catalytic activity of naked gold particles. Conte, M. Corma, A. Supported gold nanoparticles as catalysts for organic reactions. Corti, C. Commercial aspects of gold catalysis. Della Pina, C. Gold-catalyzed oxidation in organic synthesis: a promise kept. Corain, N. Toshima, and G. Selective oxidation using gold. Selective oxidation of tertiary amines on gold catalysts. Update on selective oxidation using gold. Demirel, S. Oxidation of mono- and polyalcohols with gold: comparison of carbon and ceria supported catalysts.
Use of renewables for the production of chemicals: glycerol oxidation over carbon supported gold catalysts. B Environ. Reaction kinetics and modelling of the gold catalysed glycerol oxidation. Demirel-Gulen, S. Liquid phase oxidation of glycerol over carbon supported gold catalysts. Dengel, A. Chem Soc. Dalton Trans. Derouane, E. Catalytic Activation and Functionalisation of Light Alkanes. Dordrecht: Springer. Di Nicola, C. Trinuclear triangular copper II clusters - Synthesis, electrochemical studies and catalytic peroxidative oxidation of cycloalkanes. Dimitratos, N. Selective liquid phase oxidation with supported metal nanoparticles.
Au, Pd mono and bimetallic catalysts supported on graphite using the immobilisation method: Synthesis and catalytic testing for liquid phase oxidation of glycerol. Synergetic effect of platinum or palladium on gold catalyst in the selective oxidation of D-sorbitol. Gold based bimetallic catalysts for liquid phase applications. Effect of the preparation method of supported Au nanoparticles in the liquid phase oxidation of glycerol. Dobrosz, I. Effect of the preparation of supported gold particles on the catalytic activity in CO oxidation reaction. Dobrosz-Gomez, I.
Donoeva, B. Colloidal Au catalyst preparation: selective removal of polyvinylpyrrolidone from active Au sites. Du, W. Jiang Paris: Atlantis Press , — Dudley, G. On the existence of and mechanism for microwave-specific reaction rate enhancement. Edwards, J. Nanocrystalline gold and gold-palladium as effective catalysts for selective oxidation. Emayavaramban, P. Gold nanoparticles supported on magnesium oxide nanorods for oxidation of alcohols.
Gold modified zeolite: an efficient heterogeneous catalyst for N-arylation of indazole. Enache, D. Science , — Fang, W. Synthesis and applications of triangular gold nanoplates. Fernandes, R. Applied Catalysis A: General , — Fernando, J. Controlling Au photodeposition on large ZnO nanoparticles. ACS Appl. Interfaces 8, — Ferraz, C. Oxidation of benzyl alcohol catalyzed by gold nanoparticles under alkaline conditions: weak vs. Fisher, J. Fulminating gold. Freakley, S. Gold catalysis: a reflection on where we are now.
Fristrup, P. Mechanistic investigation of the gold-catalyzed aerobic oxidation of alcohols. Fu, Q. Gold—ceria catalysts for low-temperature water-gas shift reaction. Gaiassi, A. Gold catalysts for the direct oxidation of aminoalcohols to aminoacids. Garcia, R. Chemoselective catalytic-oxidation of glycerol with air on platinum metals. Giorgi, P. Selective oxidation of activated alcohols by supported gold nanoparticles under an atmospheric pressure of O batch and continuous-flow studies. ChemCatChem 9, — Gluhoi, A. Structural and chemical promoter effects of alkali earth and cerium oxides in CO oxidation on supported gold.
Gogoi, N. TiO 2 supported gold nanoparticles: an efficient photocatalyst for oxidation of alcohol to aldehyde and ketone in presence of visible light irradiation. Gualteros, J. Synthesis of highly dispersed gold nanoparticles on Al 2 O 3 , SiO 2 , and TiO 2 for the solvent-free oxidation of benzyl alcohol under low metal loadings. Gui, Z. Guo, Z. Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry.
Haider, P. Gold supported on Mg, Al and Cu containing mixed oxides: relation between surface properties and behavior in catalytic aerobic oxidation of 1-phenylethanol. Hallett-Tapley, G. Supported gold nanoparticles as efficient catalysts in the solvent less plasmon mediated oxidation of sec-Phenethyl and benzyl alcohol. C , — Hargittai, M. Haruta, A. When gold is not noble: catalysis by nanoparticles. Haruta, M. Size- and support-dependency in the catalysis of gold. Today 36, — Catalysis of gold nanoparticles deposited on metal oxides. Gold as a novel catalyst in the 21st century: Preparation, working mechanism and applications. Catalysis - Gold rush. Nature , — Advances in the catalysis of Au nanoparticles.
Hashmi, A. Gold Catalysis. Gold catalysis in total synthesis. He, P. Preparation of Au-loaded TiO 2 by photochemical deposition and ozone photocatalytic decomposition. Surface Rev. He, Q. Population and hierarchy of active species in gold iron oxide catalysts for carbon monoxide oxidation. Switching-off toluene formation in the solvent-free oxidation of benzyl alcohol using supported trimetallic Au-Pd-Pt nanoparticles. Faraday Discuss. Hereijgers, B. Selective oxidation of methanol to hydrogen over gold catalysts promoted by alkaline-earth-metal and lanthanum oxides. ChemSusChem 2, — Aerobic oxidation of cyclohexane by gold-based catalysts: new mechanistic insight by thorough product analysis. An attempt to selectively oxidize methane over supported gold catalysts. Hidalgo, M.
Effect of sulfate pretreatment on gold-modified TiO 2 for photocatalytic applications. Photodeposition of gold on titanium dioxide for photocatalytic phenol oxidation. Hong, Y. Stars, such as our sun, generate energy through the power of fusion , where smaller elements are fused, or combined, together into heavier elements. To start with, a star may be mostly hydrogen , the smallest element. The process of fusion under immense pressure and heat in the star's core will generate helium.
When hydrogen runs low and the star begins to reach the next phase of its life cycle, it will fuse helium into the next heavier element, and so on. This process continues until the element of iron, where the balance suddenly shifts. Because fusing iron does not create energy, it consumes it, according to the University of Oregon opens in new tab. With no means of generating internal energy to counteract its own immense pressure and gravity , the star begins to collapse onto itself. If the star is large enough the result is a supernova — a massive star explosion, according to NASA opens in new tab.
Heavier elements are formed during the incredible energy generated during this process, including gold. Related: How can a star be older than the universe? Five thousand years ago, the massive Nile River was the key to the ancient Egyptian empire, according to the Australian government opens in new tab. Its water allowed a bounty of crops to be grown along its edge, keeping its citizens, and its armies, well fed. But there was also a shiny yellow metal that came running down the river, the element of gold.
The Egyptians eagerly took this visually appealing treasure and found that because it was naturally pure and malleable, it required little refinement to be turned into mesmerizing decorations. Gold as a decoration didn't stop at ancient Egypt: A Stone Age woman found buried outside of London wore a strand of gold around her neck; Celts in the third century B. Gold swiftly came to be a symbol, and unit, of wealth, and it has maintained this allure through time and around the globe. Several millennia after the Egyptian pharaohs and their tombs of gold, the Aztec Empire's gold riches were plundered by the Conquistadors who sought the valuable metal for their own.
Later still, workers flocked to Western coast of the United States to take part in the California "gold rush", seeking their own fortunes, according to National Geographic opens in new tab. Therefore gold has driven humans to diplomacy, mass migrations, and even acts of genocide. Without this metal, our history would be quite different. Gold also plays a strong role in Australian history. In the late 19th century, so many flocked to the country to take part in its booming gold rush that the population of Australia tripled. Owing to its pervasive deposits, the country is still mined for the metal today, according to the Australian government. However, one company, named Evolution Mining, found a different treasure in their hunt for gold.
When drilling into the Australian outback's surface in search of gold deposits, the miners instead unearthed sheets of stone that resembled "shatter cones," which form on the outer rims of impact craters. They followed this finding with advanced mapping techniques that allowed the team to confirm the uncovering of a 3. The amount of gold in a necklace or ring is measured on the karat scale.
Pure gold is 24 karats. Bars of gold kept in Fort Knox and elsewhere around the world are considered to be As metals are added to gold during jewelry-making, the gold becomes less fine and the number of karats drops. The word karat comes from the carob seed. In ancient Asian bazaars, the seeds were used to balance scales that measured the weight of gold. Army Garrison in Kentucky in The first shipment of gold arrived from Philadelphia in trains surrounded by military troops. Fort Knox is framed in steel with walls of concrete. Despite the defense of a ton steel door, a dirty rumor in the s suggested that the gold in Fort Knox was gone. To quell people's fears, the director of the United States Mint guided congress people and journalists through one room of the vault, and its 8-foot-tall stacks of 36, bars of gold.
The depository holds about half of U. Treasury's stored gold, according to the U. Mint opens in new tab. Each bar weighs troy ounces about Department of Treasury. One troy ounce equals about 1. The entire stockpile, as of , weighs Fort Knox held a record amount of gold on Dec. Mint reported. Other important artifacts have also "seen" the insides of Fort Knox. Other items stored there at some point in history, according to the U. Mint include: the Magna Carta; the crown, sword, scepter, orb and cape of St. Stephen, the King of Hungary. Pyrite, the inferior mineral nicknamed fool's gold, only mimics gold in looks.
Pyrite is more common, harder, and more brittle than gold. When crushed into powder, it looks greenish-black, whereas real gold powder is yellow. Pyrite contains sulfur and iron.
Jogos de video slot Geisha Story - WebJogue o caça-níquel Booming Bars dinheiro real e se divirta no Sol dailynewsflucc.free.bgg: Gold. WebSearch from Caca Niquel stock photos, pictures and royalty-free images from iStock. Find high-quality stock photos that you won't find anywhere dailynewsflucc.free.bgg: Bars. WebGarbage or Gold: Ka-Bar BK40 - YouTube This video marks a new series, Garbage or Gold. In this video I will review the Ka-Bar Becker BK Is it Garbage, or . jogos de slots Hollywood Reels
ᐈ Caça Níquel Gold in Bars™ Grátis | Jogue + Caca Niqueis!
Jogar slots Cash Detective - WebMay 1, · Pantanal Caça Niquel. R7 Developers. Contains ads In-app purchases. star. K reviews. K+ Downloads. Teen. info. Install. Add to wishlist. About this Missing: Gold · Bars. WebJogue o caça-níquel Drill That Gold - Sol Casino. LoadingMissing: Bars. WebAfter melting gold and making its bar I dicide to have a drop test in nitric acid#shorts #selfwork #gold #nitricacid. Jogos de casino gratis caca niqueis Call of Fruity
caça-níquel - Wiktionary
Video slot Pearls of India - WebThe problem of statistical characteristics of occurrence cadmium and nickel content in gallstones from active and passive smoking and no smoking women living in southern Missing: Bars. WebJogue o caça-níquel Aztec Gold Megaways - Sol Casino. LoadingMissing: Bars. WebJogue o caça-níquel Fishin' BIGGER Pots Of Gold dinheiro real e se divirta no Sol Casino. Loading Missing: Bars. Jogos de slots gratis 27 Hot Lines Deluxe Edition
Deposit Bonus - HC - % Welcome Bonus + 50% Cash Back | High Country Casino
Slots gratuitos sem deposito Wild Spirit - WebFeb 8, · The first thing to know about gold bars is that they must be of certain purity. All Credit Suisse gold bars are % pure, among the most refined of all gold on the . WebThis chapter examines cancer risk related to the mining of gold, nickel and copper. The human carcinogenicity of nickel depends upon the species of nickel, its concentration Missing: Bars. WebSearch from Caça Niquel stock photos, pictures and royalty-free images from iStock. Find high-quality stock photos that you won't find anywhere dailynewsflucc.free.bgg: Gold · Bars. Slots gratis sem download Flying Colors

How Much Is a Gold Bar Worth In ? - Better Bullion
Slots gratuitos sem deposito Frog Story - WebAn easy test to do at home would be to check whether a strong magnet affects the bar (it won’t affect a real gold bullion or coin). Try picking up your gold item with a magnet. If . Webcaça - níquel? (uncountable) (Brazil) slot machine synonym. Synonym: slot machine (Portugal) Categories: Portuguese lemmas. Portuguese nouns. Portuguese uncountable Missing: Gold · Bars. WebSep 2, · Indeed, nickel prices had a wide monthly range from to , with the price in April sitting at a low of 11, U.S. dollars per metric ton, and rising to a Missing: Bars. Jogos caca-niqueis de cassino gratis Fruit Machine 27

Credit Suisse Gold Bars: Everything You Need to Know - Metals Resource - April
Caca-niquel Hero’s Quest - WebJQK Double Towel Bar, 24 Inch Brass Gold Bath Towel Rack for Bathroom, Stainless Steel Thicken mm Towel Holder Wall Mount Brushed Gold, Total Length Inch, . WebPreview of Spotify. Sign up to get unlimited songs and podcasts with occasional ads. No credit card dailynewsflucc.free.bgg: Gold · Bars. WebSep 9, · We couldn’t believe that N!CK’S Swedish Style Snack Bar had no added sugar (and just 2g sugar overall).. The line was developed to conform with the keto† diet. Missing: Gold. Caca-niqueis online gratis Le Mystere du Prince
:strip_icc()/i.s3.glbimg.com/v1/AUTH_59edd422c0c84a879bd37670ae4f538a/internal_photos/bs/2020/L/A/u47oifSvKeaAUuyPUeBA/maquina-caca-niquel.jpeg)
Nescafé Gold Salted Caramel Mocha | Nescafé | UK & IE
Slot gratis online Tiki Shuffle - Web4, Followers, 2, Following, 24 Posts - See Instagram photos and videos from 𝙊 𝙢𝙚𝙡𝙝𝙤𝙧 𝙘𝙖ç𝙖 𝙣𝙞𝙦𝙪𝙚𝙡🤑🤑🤑 (@cacaniqueis)Missing: Gold · Bars. WebMake your next moment extra special with our limited edition NESCAFÉ GOLD Salted Caramel Mocha. Each convenient sachet contains all you need to make a deliciously . WebListen now only on Spotify: With JEFF, Lu e Luan, ryder and moreMissing: Gold · Bars. Slots gratuitos sem download e sem cadastro Burning Dice
